Home
40+ years experience providing Mechanical, Electrical, Software, and Manufacturing Engineering services to inventors, physicians, start-up companies, government agencies, and corporations.
Medical, Dental, Veterinary Products
We do not outsource Engineering to low-wage countries!
Human Health Product Design
Veterinary Health Product Design
Care, Treatment, Diagnosis Products
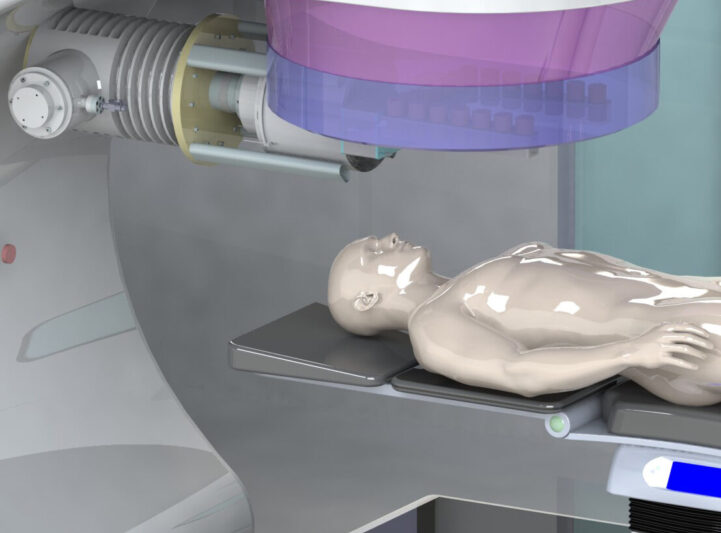
Interventional medicine
Diagnostics
Point of Care (POC)
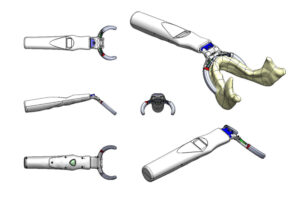
Surgical
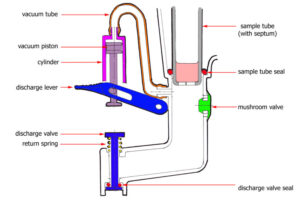
Pharmaceuticals
Drug delivery
Veterinary Care Products
Animal husbandry
Drug delivery
Risk analysis management
Design History Files
Quality plan creation
Document Control For Regulatory Submittals
FMEA and PFMEA
Human factors —- Gestalt design and inferences
Verification and validation
510k – Data Prep and Documentation For Submission
Prototyping and Manufacturing
How we do business
Select from the topics here for answers to common questions. To ask other questions please fill out our contact form.
We do not require you to sign any contract of any sort. You can stop the project at any time for a refund of any unused balance.
We do only good faith estimates based on our years of experience. The design process is just too abstract and fluid to provide fixed price quotes.
Our engineers have worked with the largest corporations in the medical and other technology fields. We know what is required for FDA submission, and whether a product must apply or not.
- Fill out our contact form
- Talk with our engineers
- Get your estimate
- Make a deposit
You do! You are the inventor, we just help you develop it into a viable product. We can assist you with the patent process. You own everything.
We are a design engineering house however we work with prototype and production manufacturers in the US, China, and Mexico. We can manage all aspects of your product production.
We have connections in other industries that you may need when bringing your product to fruition. We can refer you to Patent And Trademark Attorneys also Medical Insurance providers.